Professor Hielscher leads the recently established Department of Biomedical Engineering and directs research in his Clinical Biophotonics Laboratory (CBL). The mission of the CBL is to establish optical tomography as a viable biomedical imaging modality and transfer this technology into clinical practice. The goal is to develop a
patient-centered approach that addresses all aspects of modern precision medicine in state-of-the-art healthcare. To this end, Prof. Hielscher's team is developing cutting-edge imaging hardware and software that provide 3-dimensional distributions of physiologically relevant parameters such as oxygen saturation or total hemoglobin concentrations and more. This includes the design of wearable devices that allow continuous patient monitoring. The CBL is currently applying this emerging technology in various clinical and preclinical studies that focus on the diagnosis and monitoring breast cancer, arthritis, peripheral artery disease (PAD), and diabetic foot syndrome (DFS). Furthermore, techniques are being developed for real-time monitoring of brain activities.
Education
Los Alamos National Laboratories, Los Alamos, NM, 1995 - 1998
Postdoctoral Fellow, Division Biosciences and Biotechnology
Rice University, Houston, TX, 1991 - 1995
Ph.D., Department of Electrical and Computer Engineering
University of Hannover, Hannover, Germany, 1989 - 1991
"Diplom" (~ Master of Science), Department of Quantum Optics
Publications
- M.L. Altoe, K. Kalinsky, A. Marone, Hyun Kim, Hua Guo, H. Hibshoosh, M. Tejada, Ka. Crew, M. Accordino, M. Trivedi, D. Hershman, and A.H. Hielscher, “Effects of neoadjuvant chemotherapy on the non-tumor bearing breast assessed by diffusion optical tomography,” submitted to Breast Cancer Research, June 2020.
- Y. Kim, A. Marone, G. Danias, W. Tang, H.K. Kim, A.D. Askanase, I. Kymissis, A.H. Hielscher, “A flexible optical imaging band system for the assessment of arthritis in patients with systemic lupus erythematosus,” submitted to Advanced Healthcare Materials, June 2020.
- M.L. Altoe, K. Kalinsky, A. Marone, H.K. Kim, Hua Guo, H. Hibshoosh, M. Tejada, Ka. Crew, M. Accordino, M. Trivedi, D. Hershman, and A.H. Hielscher, “Changes in diffuse-optical-tomography images during early stages of neoadjuvant chemotherapy correlate with tumor response in different breast cancer subtypes, submitted to Clinical Cancer Research, March 2020.
- M.L. Altoe, A. Marone, H.K. Kim, K. Kalinsky, D.L. Hershman, A.H. Hielscher, and R.S. Ha, “Diffuse optical tomography of the breast: a potential modifiable biomarker of breast cancer risk with neoadjuvant chemotherapy,” Biomedical Optics Express 10(8), pp. 4305-4315 (2019). PMID: 31453012; PMCID: PMC6701514; <https://doi.org/10.1364/BOE.10.004305
- A. Marone, J. W. Hoi, M.A. Khalil, H.K. Kim, G. Shrikhande, R. Dayal, A.H. Hielscher, “Modeling of the Hemodynamics in the Feet of Patients with Peripheral Artery Disease,” Biomedical Optics Express 10(2), pp. 657-669 (2019). PMID: 30800506; PMCID: PMC6377885; <https://doi.org/10.1364/BOE.10.000657>
- A.H. Hielscher, J. Ramella-Roman, L.V. Wang, “Pioneer in Biomedical Optics: Introduction to the Special Section in Honor of Steven L. Jacques,” J. Biomedical Optics 23(12):1, December 2018. <https://doi.org/10.1117/1.JBO.23.12.121601>
- J.W. Hoi, H.K. Kim, C.J. Fong, L. Zweck, A.H. Hielscher, “Non-contact dynamic diffuse optical tomography imaging system for evaluating lower extremity vasculature,” Biomedical Optics Express, Vol. 9, No. 11, pp. 5597-5614, Oct. 19, 2018. PMID: 30460149; PMCID: PMC6238914 <https://doi.org/10.1364/BOE.9.005597>
- J.E. Gunther, E. Lim, H.K. Kim, M. Flexman, J.A. Campbell, H. Hibshoosh, K. Crew, K. Kalinsky, D.L. Hershman, A.H. Hielscher, “Dynamic diffuse optical tomography for monitoring neoadjuvant chemotherapy in breast cancer patients,” Radiology 2018 Jun; 287(3):778-786. Epub 2018 Feb 12; PMID: 29431574; PMCID: PMC5978455 [Available on 2018-06-01]; <https://doi.org/10.1148/radiol.2018161041>
- H.K. Kim, L.D. Montejo, J. Jia, A.H. Hielscher, “Frequency-domain optical tomographic image reconstruction algorithm with the simplified spherical harmonics (SP3) light propagation model,” International Journal of Thermal Science 116, 265-277, 2017. doi: 10.1016/j.ijthermalsci.2017.03.004; PMID: 29062243; PMCID: PMC5649649; <https://doi.org/10.1016/j.ijthermalsci.2017.03.004>
- C.J. Fong, M. Garzon, J.W. Hoi, H.K. Kim, C.T. Lauren, K. Morel, L. Geller, N. Antonov, N. Weitz, J. Wu, A.H. Hielscher, “Assessment of Infantile Hemangiomas Using a Handheld Wireless Diffuse Optical Spectroscopic Device, Pediatric Dermatology, 2017 May 26. doi: 10.1111/pde.13150. [Epub ahead of print] PMID: 28548465.
- E.A. Lim, J.E. Gunther, H.K. Kim, M. Flexman, H. Hibshoosh, K. Crew, B. Taback, J. Campbell, K. Kalinsky, A.H. Hielscher, D.L. Hershman, “Diffuse optical tomography changes correlate with Residual Cancer Burden after neoadjuvant chemotherapy in breast cancer patients” Breast Cancer Research and Treatment 162(3), pp. 533-540 (April 2017); PMID: 28190249; <https://doi.org/10.1007/s10549-017-4150-7> [6]
- J. Jia, H.K. Kim, A.H. Hielscher, “Fast linear solver for radiative transport equation with multiple right hand sides in diffuse optical tomography, Journal of Quantitative Spectroscopy and Radiative Transfer, Vol. 167, pp. 10-22 (Dec 1, 2015). PMID: 26345531; PMCID: PMC4556172. [9]
- M.A. Khalil, H.K. Kim, J.W. Hoi, I.-K. Kim, R. Dayal, G. Shrikhande, A.H. Hielscher, “Detection of Peripheral Arterial Disease Within the Foot Using Vascular Optical Tomographic Imaging: A Clinical Pilot Study,” European J. of Vascular and Endovascular Surgery, Vol 49(1), pp. 83–89 (2015). PMID: 25457299; PMCID: PMC4439381 <https://doi.org/10.1016/j.ejvs.2014.10.010> [26]
- J.H. Lee, H.K. Kim, C. Chandhanayingyong, F.Y. Lee FY, A.H. Hielscher, “Non-Contact Small Animal Fluorescence Imaging System for Simultaneous Multi-directional Angular-dependent Data Acquisition,” Biomedical Optics Express, Vol. 5, Iss. 7, pp. 2301–2316 (2014). <https://doi.org/10.1364/BOE.5.002301> eCollection 2014 July 1] PMID: 25071965 [13]
- M.L. Flexman, H.K. Kim, J.E. Gunther, E.A. Lim, M.C. Alvarez, E. Desperito, K. Kalinsky D.L. Hershman, A.H. Hielscher, “Optical biomarkers for breast cancer derived from dynamic diffuse optical tomography,” Journal of Biomedical Optics 18(9), 096012 (Sep 18, 2013). <https://doi.org/10.1117/1.JBO.18.9.096012> PMID: 24048367 [46]
- L.D. Montejo, J. Jia, H.K. Kim, U.J. Netz, S. Blaschke, G.A. Muller, A.H. Hielscher, “Computer Aided Diagnosis of Rheumatoid Arthritis With Optical Tomography, Part 1: Feature Extraction,” Journal of Biomedical Optics 18(7), 076001 (2013). PMID: 23856915; <https://doi.org/10.1117/1.JBO.18.7.076001> [31]
- L.D. Montejo, J. Jia, H.K. Kim, U.J. Netz, S. Blaschke, G.A. Muller, A.H. Hielscher, “Computer Aided Diagnosis of Rheumatoid Arthritis With Optical Tomography, Part 2: Image Classification,” Journal of Biomedical Optics 18(7), 076002 (2013). PMID: 23856916; <https://doi.org/10.1117/1.JBO.18.7.076002> [27]
- M. A. Khalil, H.K. Kim, I.-K.Kim, M. Flexman, R. Dayal, G. Shrikhande, and A.H. Hielscher, “Dynamic Diffuse Optical Tomography Imaging of Peripheral Arterial Disease,” Biomedical Optics Express Vol. 3, No. 9, 2288-98, September 2012. PMID: 23024920 [37]
- B.J Beattie, D.L.J Thorek, C.R. Schmidtlein, K.S. Pentlow, J.L. Humm. and A.H Hielscher, “Quantitative modeling of Cerenkov light production efficiency from medical radionuclides,” PLoS ONE 7(2):e31402. [doi:10.1371/journal.pone.0031402 (2012)] PMID: 22363636 [69]
- S. Sirsi, M. Flexman, F. Vlachos, J. Huang, S.L. Hernandez, H.K. Kim, T.J. Johung, J. Gander, A. Reichstein, B.S. Lampl, A. Wang, A.H. Hielscher, D.J. Yamashiro, J.J. Kandel, M. Borden, “Contrast Ultrasound Allows Identification of Early Responder Tumor Models in Anti-Angiogenic Therapy,” Ultrasound in Medicine and Biology Vol.38, No.6, pp.1019–1029 (2012). [doi: 10.1016/j.ultrasmedbio.2012.01.014] PMID: 22425376 [64]
- M.L. Flexman, F. Vlachos, H.K. Kim, S. Sirsi, J. Huang, S.L. Hernandez, T.J. Johung, J. Gander, A. Reichstein, B.S. Lampl, A. Wang, M. Borden, D.J. Yamashiro, J.J. Kandel, A.H. Hielscher, “Monitoring Early Tumor Response to Drug Therapy with Diffuse Optical Tomography,” Journal of Biomedical Optics 17(1), 016014 (2012). [doi: 10.1117/1.JBO.17.1.016014] PMID: 22352664 [20]
- M.L. Flexman, H.K. Kim, R. Stoll, M. Khalil, C.J. Fong, A.H. Hielscher, “A wireless handheld probe with spectrally constrained evolution strategies for diffuse optical imaging of tissue,” Review of Scientific Instruments 83(3), pp. 033108 - 033108-8 (2012). [doi: 10.1063/1.3694494] PMID: 22462907 [35]
Patents
- JH Lee, HK Kim, LIM Emerson, AH Hielscher, “Transrectal Diagnostic Device,” US Patent App. 16/303,706, published as US20200008737A1 on 2020-01-09.
- AH Hielscher, M Khalil, R Dayal, IK Parrack, HK Kim, “Dynamic Optical Tomographic Imaging Devices Methods and Systems,” US Patent App. 16/216,137, published as US20190282134A1 on 2019-09-19.
- AH Hielscher, ML Flexman, K Yeager , “Interfacing systems, devices, and methods for optical imaging,” US Patent 10,376,150, 2019. published as US10376150B2 on 2019-08-13.
- AH Hielscher, ML Flexman, R Stoll, HK Kim, “Compact Optical Imaging Devices, Systems, and Methods,” US Patent App. 16/129,925, published as US20190239751A1 on 2019-08-08.
- AH Hielscher, CJ Fong, J Hoi, HK Kim, M Khalil,” Monitoring Treatment of Peripheral Artery Disease (PAD) Using Diffuse Optical Imaging,” US Patent App. 16/093,775, published as US20190125195A1 on 2019-05-02.
- HK Kim, AH Hielscher, “Tomographic Imaging Methods, Devices, and Systems,” US Patent App. 16/211,693, published as US20190110024A1 on 2019-04-11.
- A.H. Hielscher, A. Marone, I. Kymissis, Y. Kim, A.D. Askanase, “Flexible Optical Imaging Bands and Optical Imaging Methods for the Diagnosis and Monitoring of Systemic Lupus Erythematosus in Finger Joints,” Provisional patent application No. 62/747,728 filed on October 19, 2018.
- A.H. Hielscher, H.K. Kim, L.D. Montejo, S. Blaschke, U.J. Netz, G.A. Mueller, J. Beuthan,, “Medical Imaging Devices, Methods, and Systems,” Korean Application No. 2013-7018117, based off PCT/US2011/064723 filed December 12, 2011, allowed September 27, 2018. (patent number pending)
- H.K. Kim, A.H. Hielscher, “Tomographic Imaging Methods, Devices, and Systems,“ U.S. Application No. 14/356,924 filed May 8, 2014; based from PCT/US2012/064163 filed November 8, 2012, allowed September 19, 2018. (patent number pending)
- A.H. Hielscher, C.J. Fong, J. Hoi, H.K. Kim, M. Khalil, M. Khalil, “Monitoring treatment of peripheral artery disease (pad) using diffuse optical imaging,“ PCT/US2017/029027 filed 2017-04-23, WO2017189376A1 filed 2017-11-02.
- J.H. Lee, H.K. Kim, E. Lim, A.H. Hielscher, “Transrectal Diagnostic Device,” (PTC/US2017/ 034800, WO2017205808A1), Application filed 2016-05-27, published 2017-05-26.
- A.H. Hielscher, H.K. Kim, L. D. Montejo, “Systems, methods, and devices for image reconstruction using combined PDE-constrained and simplified spherical harmonics algorithm,” (PCT/US2013/071269), US Patent No. 9,495,516 (issued November 15, 2016).
- A.H. Hielscher, M. Khalil, R. Dayal, I.-K. Kim, H.K. Kim, “Dynamic Optical Tomographic Imaging Devices, Methods, and Systems,” Atty. Docket No. T4356-19064WO01, T4356-18602US01; PCT/US2011/060489, US 13/876,861) US Patent No. 9,429,089 (issued November 15, 2016); Announcement to intention to grant by European Patent Office on June 15, 2015.
- A.H. Hielscher, H.K. Kim, L. D. Montejo, “Medical Imaging Devices, Methods, and Systems,” Atty. Docket No. T4356-18601US01, PCT/US2011/064723, US 13/993,592), US Patent No. 9,486,142 (issued November 8, 2016)
- A.H. Hielscher, C.F. Fong, “Imaging Interfaces for Full Finger and Full Hand Optical Tomography,” (Invention Report CU13042, Atty. Docket No. T4356-19064WO01, PCT/US13/54897) filed February 12, 2015; published June 4, 2015 (Publication No. 20,150,150,458)
- A.H.Hielscher, Y. Li, A. Bur, M. Flexman, J. Masciotti, C.J. Fong, “System and Methods for Dynamic Imaging of Tissue Using Digital Optical Tomography”, US Patent No. 9.037,216 (issued May 19, 2015)
- A.H. Hielscher, J.H. Lee, “Systems and Methods for Simultaneous Muli-Directional Imaging for Capturing Tomographic Data,” (CU12122 , PCT/US2012/064245, US 14/356,932,), filed November 8, 2012; National filings for US, Korea, Japan, and Europe submitted May 8, 2014; published November 6, 2014 (Publication No. 20,140,330,116)
- H.K. Kim, A.H. Hielscher, “Tomographic Imaging Methods, Devices, and Systems,” (Columbia Invention Report # CU12119, PCT/US2012/064163, Application # US 14/356,924), filed November 8, 2012; National filings for US, Korea, Japan, and Europe submitted May 8, 2014; published October 23, 2014 (Publication No. 20,140,313,305)
- A.H. Hielscher, M.L. Flexman, R. Stoll, H.K. Kim, “Compact optical imaging devices, systems, and methods,” (CU12093, PCT/US2012/058065, US 14/348,081), filed September 28, 2012; published August 28, 2014. (Publication No. 20,140,243,681)
- A.H. Hielscher, K. Yeager, M.L. Flexman, “Interfacing systems, devices, and methods for optical imaging,” (CU12094, PCT/US2012/058004, application # EP20120837252),filed September 28, 2012; published August 6, 2014 (Publication No. 20,140,236,003)
- A.H. Hielscher, H.K. Kim, L. D. Montejo, S. Blaschke, U. Netz, G.A. Mueller, J. Beuthan, “Medical Imaging Devices, Methods, and Systems,” (CU invention reports # M11-007 and M11-122, PCT/US2011/064747, US 13/993,578,) filed Dec 13, 2011; published on March 27, 2014 (Publication No. 20,140,088,415)
- J.M. Lasker, A.H. Hielscher, J.M. Masciotti, C.H. Schmitz, M. Schoenecker, "System and Methods for Digital Detection of a Tomographic Signal”, US Patent No. 7,728,986 B2 (issued June 1, 2010)
- J.M. Lasker, A.H. Hielscher, J.M. Masciotti, C.H. Schmitz, M. Schoenecker, "Digital Signal Processor Based Detection System, Method, and Apparatus for Optical Tomography” US Patent No. 7,463,362 B2 (issued December 9, 2008)
- A.H. Hielscher, J. Mourant, I. Bigio, "Characterization of highly scattering media by measurement of diffusely backscattered polarized light," US Patent No. 6,011,626 (issued January 4, 2000)
Information for Mentees
About Me:
I came to Tandon in the summer of 2021 as the new chair of the BME department. Before I was at Columbia for 20 years and chaired the Provost's Tenure Track Advisory Committee.
My Science:
Biomedical Optics, Clinical Studies, Inverse Problems
Research News
Postintervention monitoring of peripheral arterial disease wound healing using dynamic vascular optical spectroscopy
Peripheral arterial disease (PAD) is a vascular disease that is caused by clogging of the arteries due to plaque. The lower legs and feet are often impacted, and symptoms include pain, numbness, difficulty walking, and non-healing wounds.
For many patients, wounds continue not to heal even after they have undergone a surgical revascularization procedure designed to unclog the affected arteries, necessitating another intervention. Determining whether wounds will heal must be done as soon as possible after the first intervention to reduce the duration of a patient’s pain and increase the likelihood of a good outcome.
Currently, the most common methods for monitoring PAD progression (and related wound healing) are the ankle-brachial index (ABI) and ultrasound imaging. The ABI uses the ratio of systolic blood pressure measurements from arteries in the lower extremities to the systolic blood pressure measurement from the brachial artery in the arm. PAD patients generally have pressure ratios that are below a certain threshold (often 0.9). The ABI has low accuracy when monitoring vasculature in diabetic patients and ultrasound imaging has low accuracy when monitoring smaller arteries such as those in the feet. Unfortunately, PAD is often comorbid with diabetes and the affected arteries are commonly in the feet.
To address the existing limitations of the current technology, a team from NYU Tandon School of Engineering’s department of Biomedical Engineering, including lead author Nisha Maheshwari from Andreas Hielscher's Clinical Biophotonics Laboratory, developed optical imaging technology to assist physicians with monitoring the healing of lower limb ulcers after a surgical intervention.
Dynamic vascular optical spectroscopy (DVOS) is an optical imaging technology that uses light in the red and near-infrared ranges to determine characteristics of blood flow through arteries. In the paper “Postintervention monitoring of peripheral arterial disease wound healing using dynamic vascular optical spectroscopy,” published in the Journal of Biomedical Optics, the team used their DVOS system to monitor a set of 14 patients with PAD that underwent a surgical revascularization procedure. Of these patients, five needed a second intervention due to the persistence of non-healing wounds.
The team was able to correctly categorize the long-term healing and non-healing of wounds in 93% of this patient population within only one month after each patient’s initial intervention. The method outperformed the gold standard methods of ultrasound and ABI. These findings suggest that the DVOS may be able to assist physicians in improving patient outcomes and reducing long-term pain by determining wound outcome earlier than existing technology can.
The authors would like to thank the patients who volunteered for this study for their time and participation. This work was supported in part by the National Heart, Lung, and Blood Institute (Grant No. NHLBI-1R01-HL115336); Wallace H. Coulter Foundation; Society of Vascular Surgery; Columbia University Fu Foundation School of Engineering and Applied Science; and New York University Tandon School of Engineering.
"Maheshwari N, Marone A, Altoé M, Kim SHK, Bajakian DR, Hielscher AH. Postintervention monitoring of peripheral arterial disease wound healing using dynamic vascular optical spectroscopy. J Biomed Opt. 2022 Dec;27(12):125002. doi: 10.1117/1.JBO.27.12.125002. Epub 2022 Dec 24. PMID: 36582192; PMCID: PMC9789744."
Evaluation of lupus arthritis using frequency domain optical imaging
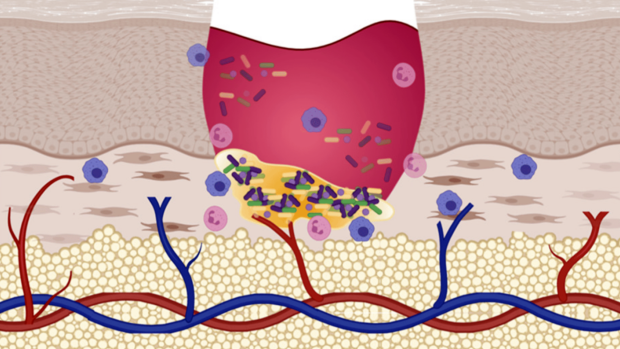
Systemic lupus erythematosus (SLE), commonly referred to as simply “lupus”, is an autoimmune disorder where the body’s immune system attacks healthy tissue. Lupus affects somewhere between 20 to 150 people per 100,000, with variations among different racial and ethnic groups. The disease often causes arthritic symptoms in the joints, which can be debilitating in some cases.
Despite the severity of the disease, identification of lupus arthritis and assessment of its activity remains a challenge in clinical practice. Evaluations based on traditional joint examination lack precision, due to its subjective nature and accuracy in situations such as obese digits and co-existing fibromyalgia. As such, these examinations have limited ability to render quantitative data about improvement and worsening.
Recently, imaging technology, especially ultrasound (US) and magnetic resonance imaging (MRI), has enabled more objective and detailed assessment of articular and periarticular abnormalities with higher sensitivity. However, MRI and US are expensive and time-consuming. Furthermore, US has been found to be very operator dependent. Therefore, both modalities are currently not routinely used in practice. There is a clear unmet need for a simple, reliable, non-invasive and low-cost imaging modality that can objectively assess and monitor arthritis progress in patients with lupus.
Now, researchers at NYU Tandon in collaboration with Columbia University are exploring optical imaging technology as a reliable way to diagnose patients and assess the progression of the disease. The researchers, including Research Assistant Professor Alessandro Marone and Chair of the Biomedical Engineering Department Andreas H. Hielscher, found that frequency domain optical imaging could reliably identify lupus arthritis, and could be used to track how the disease progressed.
The results provide strong evidence that frequency domain optical images could provide objective, accurate insights into SLE that were not possible or economically feasible using other technologies. The light diffusion identified inflammation in the blood vessels around joints, similar to but distinct from the symptoms caused by rheumatoid arthritis. With this technology, caregivers may not have to rely on patient feedback to track the progression of lupus, but can see it in action.
Optical imaging methods have been used in studies comparing osteoarthritis, rheumatoid arthritis (RA) and healthy controls. The results of those studies highlighted that patients suffering from RA have higher light absorption in the joint space compared with healthy subjects. This is likely due to the presence of inflammatory synovial fluid that decreases light transmission through the inflamed joints. But these observations have never been used to study lupus before, and these findings could provide a reliable, rapid, and cost-effective method of assessing joint involvement in lupus patients.