Jin Kim Montclare is a Professor in the Department of Chemical and Biomolecular Engineering, who is performing groundbreaking research in engineering proteins to mimic nature and, in some cases, work better than nature. She works to customize artificial proteins with the aim of targeting human disorders, drug delivery and tissue regeneration as well as create nanomaterials for electronics. Using multidisciplinary expertise in chemistry and genetic engineering, these results have already been realized.
Prior to joining NYU-Tandon, Montclare was a postdoctoral fellow at the California Institute of Technology in the Division of Chemistry and Chemical Engineering.
She received a Bachelor of Science in Chemistry from Fordham University in 1997, a Master of Science and a Ph.D. in Bioorganic Chemistry from Yale University in 2001 and 2003, respectively.
Among her many honors and awards are the AAAS Leshner Fellowship, AIMBE Fellow, ACS Rising Star Award, Agnes Faye Morgan Research Award from Iota Sigma Pi, Executive Leadership in Academic Technology and Engineering Fellowship, American Chemical Society PROGRESS /Dreyfus Lectureship, the Dreyfus Special Grants Program Award, the Air Force Office of Scientific Research Young Investigator Award, the Wechsler Award for Excellence, the Othmer Junior Fellow Award, the National Institute’s of Health Postdoctoral Fellowship, and the National Science Foundation Pre-doctoral Fellowship.
Montclare is the author of numerous papers for refereed journals, colloquia, and seminars and holds several patents.
She is a member of the American Chemical Society, the International Society for Pharmaceutical Engineering, the Biophysical Society, the Materials Research Society, the Biochemical Society, the Protein Society and American Association of Cancer Research, and the American Institute of Chemical Engineers.
Associated websites and social media
Education
Yale University, 2003
MS, Ph.D., Bioorganic Chemistry
Fordham University, 1997
BS, Chemistry
Experience
NYU Tandon School of Engineering
Professor
The Protein Engineering and Molecular Design Lab began July 2005. Broadly, our lab is focused on engineering macromolecules. The long-term goal of our lab research is to be able to predictably design or engineer artificial therapeutics, biocatalysts, scaffolds and cells. We seek to provide biologically inspired solutions to address the challenges of human disorder treatment and medicine, sustainable energy and environmental remediation.
From: July 2005 to present
California Institute of Technology
Postdoctoral fellow
Worked in the laboratory of Professor David Tirrell on evolving proteins bearing unnatural amino acids.
From: January 2003 to July 2005
Publications
Journal Articles
-
- Lindsay K. Hill,† Michael Meleties,† Xuan Xie, Erika Delgado-Fukushima, Teeba Jihad, Che Fu Liu, Sean O’Neill, Raymond S. Tu, P. Douglas Renfrew, Richard Bonneau, Youssef Z. Wadghiri & Jin K. Montclare. Thermoresponsive Protein-Engineered Coiled-coil Hydrogel for Sustained Small Molecule Release. Biomacromolecules (2019) 20, 3340-3351. DOI: https://doi.org/10.1021/acs.biomac.9b00107
- Priya Katyal, Michael Meleties & Jin K. Montclare. Self-Assembled Protein and Peptide Nanomaterials. ACS Biomaterials Science & Engineering (2019) https://doi.org/10.1021/acsbiomaterials.9b00408
- Kamia Punia, Jacob Kronenberg & Jin K. Montclare. Protein Materials for Theranostic Applications. Molecular Systems Design & Engineering (2019) 4, 1074 – 1094. doi: 10.1039/C9ME00143C
- Lindsay K. Hill, Joseph A. Frezzo, Priya Katyal, Dung Minh Hoang, Zakia Ben Youss Gironda, Cynthia Xu, Xuan Xie, Erika Delgado-Fukushima, Youssef Z. Wadghiri & Jin K. Montclare. Protein Engineered Nanoscale Micelles for Dynamic 19F Magnetic Resonance and Therapeutic Drug Delivery. ACS Nano (2019) doi:10.1021/acsnano.8b07481
- Yao Wang, Priya Katyal & Jin K. Montclare. Protein Engineered Functional Materials. Advanced Healthcare Materials (2019) doi: 10.1002/adhm.201801374.
- Hironori Kasai, Kenji Inoue, Kentaro Imamura, Carlo Yuvienco, Jin K Montclare & Seiichi Yamano. Efficient siRNA delivery and gene silencing using a lipopolypeptide hybrid vector mediated by a caveolae-mediated and temperature-dependent endocytic pathway. Journal of Nanobiotechnology (2019) 17:11 https://doi.org/10.1186/s12951-019-0444-8
- Liming Yin, Albert S. Agustinus, Carlo Yuvienco, Takeshi Minashima, Nicole L. Schnabel, Thorsten Kirsch & Jin K. Montclare. Engineered Coiled-Coil Protein for Delivery of Inverse Agonist for Osteoarthritis. Biomacromolecules (2018) 19, 1614-1624. DOI: 10.1021/acs.biomac.8b00158
- Andrew J. Olsen, Jennifer S. Haghpanah, Priya Katyal, Matthew B. Kubilius, Ruipeng Li, Nicole L. Schnabel, Sean C. O’Neill, Yao Wang, Min Dai, Navjot Singh, Raymond S. Tu & Jin K. Montclare. Protein Engineered Triblock Polymers Comprised of Two SADs: Enhanced Mechanical Properties and Binding Abilities. Biomacromolecules (2018) 19, 1552-1561. DOI: 10.1021/acs.biomac.7b01259
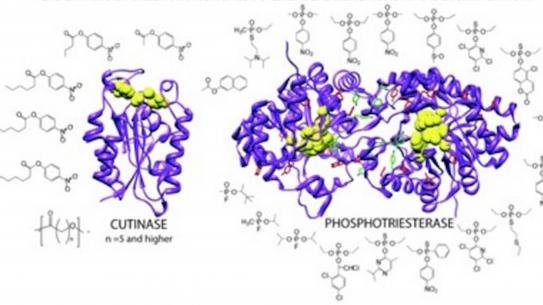
- Andrew J. Olsen, Leif A. Halvorsen, Ching-Yao Yang, Roni Barak Ventura, Liming Yin, P. Douglas Renfrew,, Richard Bonneau & Jin K. Montclare. Impact of Phenylalanines Outside the Dimer Interface on Phophotriesterase Stability and Function. Mol BioSys (2017) 13, 2092-2106. DOI: 10.1039/c7mb00196g
- Che Fu Liu, Raymond Chen, Joseph A. Frezzo, Priya Katyal, Lindsay K. Hill, Liming Yin, Nikita Srivastava, Haresh T. More, P. Douglas Renfrew, Richard Bonneau & Jin K. Montclare. Efficient dual siRNA and drug delivery using engineered lipoproteoplexes. Biomacromolecules (2017) 18, 2688-2698. DOI: 10.1021/acs.biomac.7b00203.
- Liming Yin, Carlo Yuvienco & Jin K. Montclare. Protein based therapeutic delivery agents: Contemporary developments and challenges. Biomaterials (2017) 134, 91-116. DOI: https://doi.org/10.1016/j.biomaterials.2017.04.036
- Piul S Rabbani, Anna Zhou, Zachary M Borab, Joseph A Frezzo, Nikita Srivastava, Haresh T More, William J Rifkin, Joshua A David, Samuel J Berens, Raymond Chen, Sophia Hameedi, Muhammad H Junejo, Camille Kim, Rita A Sartor, Che F Liu, Pierre B Saadeh, Jin K Montclare & Daniel J Ceradini. Novel lipoproteoplex delivers Keap1 siRNA based gene therapy to accelerate diabetic wound healing. Biomaterials (2017) 132, 1-15. DOI: http://dx.doi.org/10.1016/j.biomaterials.2017.04.001
- Min Dai,† Joseph A. Frezzo,† Etka Sharma, Raymond Chen, Navjot Singh, Carlo Yuvienco, Elif Caglar, Shu Xiao, Anjana Saxena & Jin K. Montclare. Engineered Protein Polymer-Gold Nanoparticle Hybrid Materials for Small Molecule Delivery. J Nanomed Nanotechnol (2016) 7:356. doi:10.4172/2157-7439.1000356
- Jasmin Hume, Raymond Chen, Rudy Jacquet, Michael Yang & Jin K. Montclare. Tunable Conformation Dependent Protein•Gold Nanoparticle Nanocomposites. Biomacromolecules (2015) 16, 1706-1713. DOI: 10.1021/acs.biomac.5b00098
- Joseph A. Frezzo & Jin K. Montclare. Exploring the potential of engineered coiled-coil protein microfibers in drug delivery. Therapeutic Delivery (2015) 6, 643-646.
- Haresh T. More, Kevin S. Zhang, Nikita Srivastiva, Joseph A. Frezzo & Jin K. Montclare. Influence of Fluorination on Protein Engineered Coiled-coil Fibers. Biomacromolecules (2015) 16, 1210–1217. DOI: 10.1021/bm5019062
- Jasmin Hume, Jennifer Sun, Rudy Jacquet, P. Douglas Renfrew, Jesse A. Martin, Richard Bonneau, M. Lane Gilchrist & Jin K. Montclare. Engineered Coiled-Coil Protein Microfibers. Biomacromolecules (2014) 15, 3503-3510. DOI: 10.1021/bm5004948
- Ching-Yao Yang, P. Douglas Renfrew, Andrew J. Olsen, Michelle Zhang, Carlo Yuvienco, Richard Bonneau, Jin K. Montclare. Improved Stability and Half-life of Fluorinated Phosphotriesterase using Rosetta. ChemBioChem (2014) 15, 1761-1764.
- Haresh T. More, Joseph A. Frezzo, Jisen Dai, Seiichi Yamano, Jin K. Montclare. Efficient Gene Delivery from Supercharged Coiled-coil Protein and Cationic Lipid Nanocomplexes. Biomaterials (2014) 35, 7188-7193.
- Seiichi Yamano, Jisen Dai, Shigeru Hanatani, Ken Haku, Takuto Yamanaka, Mika Ishioka, Tadahiro Takayama, Carlo Yuvienco, Sachin Khapli, Amr M. Moursi, Jin K. Montclare. Long-term efficient gene delivery using polyethylenimine with modified Tat peptide. Biomaterials (2014) 34, 1705-1715.
- Jennifer S. Haghpanah, Raymond Tu, Sandra Da Silva, Deng Yan, Silvana Mueller, Christoph Weder, E. Johan Foster, Iulia Sacui, Jeffery W. Gilman, Jin K. Montclare. Bionanocomposites: Differential Effects of Cellulose Nanocrystals on Protein Diblock Copoloymers. Biomacromolecules (2013) 14, 4360-4367.
- Daniel Yoo, Nick Tovar, Ryo Jimbo, Charles Marin, Rodolfo Anchieta, Lucas Machado, Jin Montclare, Fernando Guastaldi, Malvin Janal & Paulo Coelho, Increased Osseointegration Effect of BMP-2 on Dental Implants: An In Vivo Study. Clinical Implant Dentistry and Related Research. (2013) doi: 10.1002/jbm.a.34862
- Herbert Lannon, Jennifer S. Haghpanah, Jin K. Montclare, Eric Vanden-Eijnden & Jasna Brujic. Force-clamp experiments reveal the free energy profile and diffusion coefficient of the collapse of proteins. Phys, Rev. Lett. (2013) 110, 128301-6. doi:10.1103/PhysRevLett.110.128301
- Nancy Hom, Kinjal R. Mehta, Tsengming Chou, Amy B. Foraker, Frances M. Brodsky, Kent Kirshenbaum & Jin K. Montclare. Anisotropic nanocrystal arrays organized on protein lattices formed by recombinant clathrin fragments. J. Mat. Chem. (2012) 22, 23335-23339.
- Carlo Yuvienco, Haresh T. More, Jennifer S. Haghpanah, Raymond S. Tu & Jin K. Montclare, Modulating Supramolecular Assemblies and Mechanical Properties of Engineered Protein Materials by Fluorinated Amino Acids. Biomacromolecules. (2012) 13, 2273-2278.
- Susheel K. Gunasekar, Luona Anjia, Hiroshi Matsui & Jin K. Montclare. Effects of Divalent Metals on Nanoscopic Fiber Formation and Small Molecule Recognition of Helical Proteins. Adv. Funct. Mat. (2012) 22, 2154-2159.
- Peter J. Baker, Yan M. Chan, Moritz Hertel & Jin K. Montclare. Characterization and Identification of Protein Partners of Fn3 Domain in FnTm2. Protein Expression and Purification (2012) 81, 42-48.
- Peter J. Baker, Christopher S. Poultney, Zhiqiang Liu, Richard Gross & Jin K. Montclare. Identification and Comparison of Cutinases for Synthetic Polyester Degradation. Applied Microbiology and Biotechnology (2012) 93, 229-240.
- Min Dai, jennifer S. Haghpanah, Navjot Singh, Eric W. Roth, Alice Liang, Raymond S. Tu & Jin K. Montclare, Artificial Protein Block Polymer Libraries Bearing Two SADs: Effects of Elastin Domain Repeats. Biomacromolecules. (2011) 12, 4240-4246.
- Kinjal R. Mehta, Ching-Yao Yang & Jin K. Montclare, Modulating substrate specificity of histone acetyltransferase with unnatural amino acids. Mol. BioSyst. (2011) 7, 3050-3055
- Peter J. Baker & Jin K. Montclare, Enhanced refoldability and thermoactivity of fluorinated phosphotriesterase. Chembiochem, (2011) 12, 1845-1848.
- Seiichi Yamano, Jisen Dai, Carlo Yuvienco, Sachin Khapli, Amr M. Moursi & Jin K. Montclare. Modified Tat peptide with lipids enhances gene transfection efficiency via temperature-dependent and caveolae-mediated endocytosis. J. Contr. Rel. (2011) 152, 278-285.
- Jennifer S. Haghpanah, Carlo Yuvienco, Eric W. Roth, Alice Liang, Raymond S. Tu & Jin K. Montclare, Supramolecular Assembly and Small Molecule Recognition by Genetically Engineered Protein Block Polymers Composed of Two SADs. Mol. BioSys. (2010) 6, 1662-1667.
- Kinjal R. Mehta, Yan M. Chan+, Man X. Lee+, Ching Yao Yang, Natalya Voloshchuk & Jin K. Montclare. Mutagenesis of tGCN5 core region reveals two critical surface residues F90 and R140. Biochem. Biophys. Res. Comm. (2010) 400, 363-368.
- Natalya Voloshchuk & Jin K. Montclare, Incorporation of unnatural amino acids for synthetic biology. Mol. BioSys. (2010) 6, 65-80.
- Jennifer S. Haghpanah, Carlo Yuvienco, Deniz E. Civay, Hanna Barra, Peter J. Baker, Sachin Khapli, Natalya Voloshchuk, Susheel K. Gunasekar, Murugappan Muthukumar & Jin K. Montclare, Artificial protein block copolymers comprised of two self-assembling domains. ChemBioChem. (2009) 10, 2733-2735. *Highlighted in Futurity* http://futurity.org/
- Zhiqiang Liu, Yuying Gosser, Peter James Baker, Yaniv Ravee, Ziying Lu, Girum Alemu, Huiguang Li, Glenn L. Butterfoss, Xiang-Peng Kong, Richard Gross & Jin K. Montclare, Structural and functional studies of A. oryzae cutinase: Enhanced thermostability and hydrolytic activity of synthetic ester and polyester degradation. J. Am. Chem Soc. (2009) 131, 15711-15716.
- Susheel K. Gunasekar, Mukta Asnani, Chandani Limbad, Jennifer S. Haghpanah, Wendy Hom, Hanna Barra, Soumya Nanda & Jin K. Montclare, N-terminal aliphatic residues dictate the structure, stability and assembly of the coiled-coil region of COMP. Biochemistry. (2009) 48, 8559–8567.
- Natalya Voloshchuk, Anita Yuhua Zhu, David Snydacker & Jin K. Montclare, Positional effects of monofluorinated phenylalanines on histone acetyltransferase stability and activity. Bioorg. Med. Chem. Lett. (2009) 19, 5449-5451.
- Sachin Khapli, Jin R. Kim, Jin K. Montclare, Rastislav Levicky, Mauricio Porfiri and Stavroula Sofou. Frozen cyclohexane-in-water emulsion as a sacrificial template for the synthesis of multilayered polyelectrolyte microcapsules. Langmuir, (2009) 17, 9728-9733.
- Jin K. Montclare*, Soojin Son*, Ginevra Clark, Krishna Kumar & David A. Tirrell, Biosynthesis of stable dimeric coiled-coils bearing (2S, 4R)-5’,5’,5’-trifluoroleucine and (2S, 4S)-5’,5’,5’-trifluoroleucine. ChemBioChem (2009) 10, 84-86. * these authors contributed equally
- Susheel K. Gunasekar, Jennifer S. Haghpanah & Jin K. Montclare, Assembly of bioinspired protein fibers. Polymers for Advanced Technology. (2008) 19, 454-468.
- Natalya Voloshchuk, Man Xia Lee, Wan Wen Zhu, Ismet Caglar Tanrikulu & Jin K. Montclare, Fluorinated chloramphenicol acetyltransferase thermostability and activity profile: improved thermostability by a single-isoleucine mutant. Bioorganic Medicinal Chemistry Letters (2007) 17, 5907-5911.
- Tatyana Panchenko, Wan Wen Zhu & Jin K. Montclare, Influence of global fluorination on chloramphenicol acetyltransferase activity and stability. Biotechnology and Bioengineering (2006) 94, 921-930.
- Jin K. Montclare, Soojin Son, Ginevra Clark, Krishna Kumar & David A. Tirrell, Biosynthesis of stable dimeric coiled-coils bearing (2S, 4R)-5’,5’,5’-trifluoroleucine and (2S, 4S)-5’,5’,5’-trifluoroleucine. ChemBioChem (2009) 10, 84-86.
- Jin K. Montclare & David A. Tirrell, Evolving proteins of novel composition. Angewandte Chemie International Edition (2006) 45, 4518-4521.
- Jin K. Montclare & Alanna Schepartz, Miniature homeodomains: High specificity without an N-terminal arm. J. Am. Chem. Soc. (2003) 125, 3416-3417.
- Jin K. Montclare, Leslie S. Sloan & A. Schepartz, Electrostatic control of half-site spacing preferences by the cyclic AMP Response Element Binding Protein: Selectivity at the expense of affinity. Nucleic Acids Res. (2001) 29, 3311-3319.
Authored/Edited Books
Joseph A. Frezzo & Jin K. Montclare, Natural Composite Systems for Bioinspired Materials. In Protein-based Engineered Nanostructures (2015) Edited by T. Z. Groves and A. L. Cortajarena. In press.
Haresh T. More, Ching-Yao Yang & Jin K. Montclare, Post-Translational Modification of Proteins Incorporating Non-natural Amino Acids. In Functional Polymers by Post-Polymerization Modification: Concepts, Practical Guidelines and Applications (2012) Edited by H. A. Klok and P. Theato.
Peter James Baker, Jennifer S. Haghpanah & Jin K. Montclare Elastin-basted Protein Polymers. In Polymer Biocatalysts and Biomaterials II (2008) Edited by H. N. Cheng and R. A. Gross. Chapter 3.
Peter J. Baker & Jin K. Montclare, Biotransformations using Cutinase. In Green Polymer Chemistry: Biocatalysis and Biomaterials (2010) Edited by H. N. Cheng and R. A. Gross. 1043, 141-158.
Other Publications
Priya Chacko, Sarah Appelbaum, Heejoo Kim, Jinhui Zhao & Jin K. Montclare. Integrating Technology in STEM Education. J. Tech. Sci. Ed. (2015) 5, 5-14.
Heejoo Kim, Priya Chacko, Jinhui Zhao & Jin K. Montclare. Using Touch-Screen Technology, Apps, and Blogs To Engage and Sustain High School Students’ Interest in Chemistry Topics. J. Chem. Ed. (2014) 91, 1818-1822. doi: 10.1021/ed500234z.
Maurica S. Lewis, Jinhui Zhao & Jin K. Montclare. Development and Implementation of High School Chemistry Modules using Touch-Screen Technologies. J. Chem. Ed. (2012) 89, 1012-1018.
Robert Lorenzini, Maurica S. Lewis & Jin K. Montclare. College-Mentored Polymer/Materials Science Modules for Middle and High School Students. J. Chem. Ed. (2011) 88, 1105-1108.
Yan M. Chan, Wendy Hom & Jin K. Montclare. Mentored Chem-Bio Technology Lab to Promote Early Interest in Science. J. Chem. Ed. (2011) 88, 751-754.
Affiliations
General/Collaborative Research
- CBAS group: Jin Kim, Rasti Levicky, Maurizio Porfiri, NYU-Poly
- Thorsten Kirsch, NYU Med
- Daniel Ceradini, NYU Med
- Vikram Kapila, NYU-Poly
- Sungheon Kim, NYU Med
- Komal Jhaveri, NYU Med
- Youssef Wadghiri, NYU Med
- Kent Kirshenbaum, Chemistry, NYU
- Jasna Brujic, Physics, NYU
- Xiang-Peng Kong, NYU Med
- Seiichi Yamano, NYU Dental
- Paulo Coelho, NYU Dental
Affiliations
SUNY Downstate Medical Center Rockefeller University
NYU Chemistry
Awards
- 2016 ACS WCC Rising Star Award
- 2015 Agnes Fay Morgan Research Award from Iota Sigma Pi, National Honor Society for Women in Chemistry
- 2014 Distinguished Award for Excellence, Dedication to Invention, Innovation and Entrepreneurship
- 2014 Executive Leadership in Academic Technology and Engineering Fellow
- 2013 NSF I-Corps
- 2009 Young Observer Fellowship (declined)
- 2008 ACS PROGRESS/Dreyfus Lectureship
- 2008 Dreyfus Special Grants Program Award
- 2007 AFOSR Young Investigator Award
- 2006 Wechsler Award for Excellence
- 2006 Othmer Junior Fellow, Othmer Institute
- 2003-2005 National Institute of Health Postdoctoral Fellowship
- 2001 T. F. Cooke Teaching Award for Organic Chemistry; Yale University
- 1997-2000 National Science Foundation Graduate Research Fellowship
- 1997-2000 Pfizer Fellowship
Grants
Engineered Protein-Lipid Systems for siRNA and Small Molecule Delivery, (PI)
National Science Foundation, 09/01/15-08/31/18
Patterned protein and hybrid materials: responsive ‘chemomechanical’ shape-shifters, (PI)
Army Research Offcie, 08/01/15-07/31/18
PFI-AIR: Prototyping a Gene Transfection Tool, GeneTrain, (PI)
National Science Foundation, 08/01/14-12/31/15
Bottom-up Assembly of Engineered Protein Fibers, (PI)
Army Research Office, 10/01/11-09/30/14
Engineered Protein-Based Multi-Functional Materials, (PI)
National Science Foundation, 09/01/12-08/31/15
Computational Studies of Histone Modifications, (co-I)
National Institute of Health, 07/01/13-06/30/17
Engineered Protein Polymers, (PI)
AFOSR, 2007-10
Information for Mentees
Mentoring Style: informal and can listen and provide whatever feedback is needed; member of T&P Committee
My Research: Protein Engineering, Biomaterials, Diversity in Entrepreneurship
Research News
Engineered Multivalent Self-Assembled Binder Protein Against SARS-CoV-2 RBD
Since it appeared in 2019, COVID-19 has claimed over 6 million lives and upended society across the globe. The condition, caused by the SARS-CoV-2 virus, attacks cells in the lungs, heart and brain, among other organs. Researchers soon realized that the disease affected these organs so dramatically because its distinctive spikes binded to the angiotensin-converting enzyme 2, or ACE2 receptor. The protein - common in those organs - provides the entry point for the coronavirus to hook into and infect cells.
ACE2 receptors were thus the obvious choice when testing for or treating COVID-19. By recreating the ACE2 and introducing it to an infected body, the virus would bind to the protein, revealing itself in a test or occupying itself with a ‘fake’ receptor. But relying on the ACE2 protein alone may not provide sufficient binding to find and fight the virus.
Now, researchers from across NYU and led by Jin Kim Montclare, Professor of Chemical and Biomolecular Engineering at NYU Tandon, have created a new protein that has an increased ability to bind to viruses, creating a more efficient tool in the fight against COVID-19. The secret is creating a version of ACE2 that mimics a multivalent assembled protein (MAP). Multivalent assembled proteins are like naturally occurring antibodies. Their bodies have multiple sites that can link and bind to the viruses they are trying to attack, making them far more effective at hooking into their targets.
The ACE-MAP the team designed utilizes a coil-shaped cartilage oligomeric matrix protein, a nanomaterial that Montclare’s lab has used before in different applications. When fused with part of ACE2 across the coils surface, they found that the new materials greatly increased the valency compared to ACE2 alone, potentially binding to multiple virus bodies at a time rather than a single one.
This new material has potential uses in both detection and treatment. Because the biomaterial is so much more effective at attaching themselves to viral bodies, it would require fewer of them compared to the natural antibodies currently used in tests and therapeutics. This technology has possible uses in testing for and treating other diseases with known receptors and a similar structure, such as HIV. Ongoing research will confirm the effectiveness of ACE-MAP in other models, and may be a key component of the fight against COVID-19 in the future.
This work was supported by the National Science Foundation and the Army Research Office.
Self-assembly of stimuli-responsive coiled-coil fibrous hydrogels
Jin Kim Montclare, professor of chemical and biomolecular engineering, with affilations at NYU Langone Health and NYU College of Dentistry, directed this research with first author Michael Meleties, fellow Ph.D. student Dustin Britton, postdoctoral associate Priya Katyal, and undergraduate research assistant Bonnie Lin.
Owing to their tunable properties, hydrogels comprising stimuli-sensitive polymers are among the most appealing molecular scaffolds because their versatility allows for applications in tissue engineering, drug delivery and other biomedical fields.
Peptides and proteins are increasingly popular as building blocks because they can be stimulated to self-assemble into nanostructures such as nanoparticles or nanofibers, which enables gelation — the formation of supramolecular hydrogels that can trap water and small molecules. Engineers, to generate such smart biomaterials, are developing systems that can respond to a multitude of stimuli including heat. Although thermosensitive hydrogels are among widely studied and well-understood class of protein biomaterials, substantial progress is also reportedly being made in incorporating stimuli-responsiveness including pH, light, ionic strength, redox, as well as the addition of small molecules.
The NYU Tandon researchers, who previously reported a responsive hydrogel formed using a coiled-coil protein, Q, expanded their studies to identify the gelation of Q protein at distinct temperatures and pH conditions.
Using transmission electron microscopy, rheology and structural analyses, they observed that Q self-assembles and forms fiber-based hydrogels exhibiting upper critical solution temperature (UCST) behavior with increased elastic properties at pH 7.4 and pH 10. At pH 6, however, Q forms polydisperse nanoparticles, which do not further self-assemble and undergo gelation. The high net positive charge of Q at pH 6 creates significant electrostatic repulsion, preventing its gelation. This study will potentially guide the development of novel scaffolds and functional biomaterials that are sensitive towards biologically relevant stimuli
Montclare explained that upper critical solution temperature (UCST) phase behavior is characterized by a solution that will form a hydrogel when it is cooled below a critical temperature.
"In our case, it is due to the physical crosslinking/entanglement of fibers that our fiber-based hydrogel forms when cooled," she said, adding that when the temperature is raised above the critical temperature, the hydrogel transitions back into solution and most of the fibers should disentangle.
"In our study, we looked at how this process is affected by pH. We believe that the high net charge of the protein at pH 6 creates electrostatic repulsions that prevent the protein from assembling into fibers and further into hydrogels, while at higher pH where there would be less electrostatic repulsion, the protein is able to assemble into fibers that can then undergo gelation."
Effect of Divalent Metal Cations on the Conformation, Elastic Behavior, and Controlled Release of a Photocrosslinked Protein Engineered Hydrogel
This research was conducted by Jin Kim Montclare, Professor of Chemical and Biomolecular Engineering; and former students Yao Wang, a recent Ph. D. graduate, and Xiaole Wang, a former M.S. student.
Protein hydrogels are versatile 3-dimensional macromolecular structures with an astonishing variety of potential applications, many of them in medicine, including tissue engineering and wound healing. Because of their hydrophilic properties and internal architecture, these compounds can even trap and deliver drugs directly to targets, opening up a host of potential applications involving safe delivery of cytotoxic compounds that are standard treatment for cancer and other diseases.
To have such “Swiss Army Knife” capabilities, these typically soft materials must be imbued with properties conferring static and dynamic mechanical strength that enables them to carry a molecular payload and know when to release it.
Taking up this challenge, Montclare and her former students built upon recent work developing a photo-crosslinkable triblock copolymer protein hydrogel called CEC-D, a compound with limited viscoelastic mechanical and moderate sustained release properties. In the new work they explored the potential of transition metal cations (positively charged ions) to enhance the mechanical properties of CED-D, including its ability to encapsulate and release the small molecule curcumin, known for its anti-inflammatory properties.
In the paper, “Effect of Divalent Metal Cations on the Conformation, Elastic Behavior, and Controlled Release of a Photocrosslinked Protein Engineered Hydrogel,” published in the ACS publication Applied Bio Materials, the investigators found that the hydrogels coordinated with divalent metal ions such as Zn2+, Cu2+, and Ni2+ demonstrated control over the encapsulation and release of curcumin, a discovery suggesting that cation-tuned hydrogels constitute a promising drug delivery platform with tunable physicochemical properties.
“Depending on the metal, we can control the structure, mechanical stiffness and small molecule delivery of the hydrogel,” said Montclare. “This has important implications for drug delivery and this knowledge can be used to tailor vehicles to deliver specific therapeutics. For example, we can tailor these materials to fabricate wound dressings that improve healing by triggering drug release in the presence of metals.”
Free-Standing Photocrosslinked Protein Polymer Hydrogels for Sustained Drug Release
This research was conducted by Jin Kim Montclare, Professor of Chemical and Biomolecular Engineering; and former students Yao Wang, a recent Ph. D. graduate, and Xiaole Wang, a former M.S. student.
Protein hydrogels, 3-dimensional macromolecular structures that do not dissolve in water (in spite of being hydrophilic), can hold large quantities of aqueous solutions due to the network formed from chemical or physical crosslinking. Partly because of this they have many medical applications including tissue engineering, wound healing and drug delivery.
These materials can be synthesized by crosslinking polymers chemically via covalent bonds, or physically via non-covalent interactions, or a mixture of both. One way of doing this is through photo-initiated crosslinking, wherein chemically inert groups become photo-reactive once exposed to certain wavelengths of light.
“The advantage of employing photochemical reactive groups over traditional chemical reagents is that they give the user spatiotemporal control over polymerization,“ said Montclare. “In other words, the biopolymers bearing the photocrosslinkers can be generated and crosslinked at some later step under various conditions to achieve control over encapsulation and release of therapeutic agents.”
In this research, the team designed a macromolecular triblock polymer comprising two different self-assembling domains derived from elastin protein (E) and coiled-coil protein (C), that can be photopolymerized due to an a NHS-diazirine (D) crosslinker to produce a CEC-D hydrogel.
In the work, “Free-Standing Photocrosslinked Protein Polymer Hydrogels for Sustained Drug Release,” in Biomacromolecules, a publication of the American Chemical Society, the investigators determined the best photocrosslinker concentration and exposure time necessary to create this independent hydrogel.
The researchers found that, overall, CEC-D hydrogel exhibits comparable characteristics, including stability, drug release profile, and elastic behavior to other hydrogels.
Because it can be used for drug delivery with high encapsulation and a low but significant release of curcumin, CEC-D has been proven to be capable of a sustained release of a given drug over a week's time.