Weiqiang Chen
,
Ph.D.
-
Associate Professor of Mechanical and Aerospace Engineering and Biomedical Engineering

Weiqiang Chen is an Associate Professor of Mechanical and Aerospace Engineering and Biomedical Engineering. He received his B.S. in Physics from Nanjing University in 2005 and M.S. degrees from Shanghai Jiao Tong University in 2008 and Purdue University in 2009, both in Electrical Engineering. He earned his Ph.D. in Mechanical Engineering from the University of Michigan in 2014. Chen’s research interests focus on Lab-on-a-Chip, Organ-on-Chip, biomaterials, mechanobiology, stem cell biology, cancer biology, systems immunology, and applying microfabrication technology to illuminate biological systems at both the molecular and cellular levels.
Education
Nanjing University, 2005
Bachelor of Science, Physics
Shanghai Jiao Tong University, 2008
Master of Science, Electrical Engineering
Purdue University, 2009
Master of Science, Electrical and Computer Engineering
University of Michigan, 2014
Doctor of Philosophy, Mechanical Engineering
Experience
New York University, September 2020 to present
Associate Professor of Biomedical Engineering
NYU Langone Laura and Isaac Perlmutter Cancer Center, December 2019 to present
Faculty Member of Tumor Immunology Research Program
New York University, September 2018 to August 2020
Assistant Professor of Biomedical Engineering
New York University, September 2014 to August 2020
Assistant Professor of Mechanical Engineering
Awards
- Chemical Communications Emerging Investigator Award, 2021.
- Healthy Longevity Award, New York Academy of Sciences and Japan Agency for Medical Research and Development, 2021.
- Biomedical Engineering Society Cellular and Molecular Bioengineering Rising Star Award, 2021.
- The Interstellar Initiative Early Career Investigators, New York Academy of Sciences and Japan Agency for Medical Research and Development, 2020.
- Best Paper Award, The 10th Annual Meeting of Asian Cellular Therapy Organization, Sapporo, Japan, 2019.
- Chroma Young Investigator Award in Biomedical Engineering, 2019.
- Biomedical Engineering Society Cellular and Molecular Bioengineering Young Innovator Award, 2019.
- National Institute of General Medical Sciences Maximizing Investigators' Research Award (NIH Outstanding Investigator Award), 2019.
- Lab on a Chip Emerging Investigator, 2018.
- National Institute of Biomedical Imaging and Bioengineering Trailblazer Award, 2018.
- NYU Goddard Junior Faculty Fellowship, New York University, 2017.
- NYU Whitehead Fellowship for Junior Faculty in Biomedical and Biological Sciences, New York University, 2017.
- American Heart Association Scientist Development Award, American Heart Association, 2016.
- ProQuest Distinguished Dissertation Award, University of Michigan, 2014.
- Richard and Eleanor Towner Prize for Outstanding PhD Research, College of Engineering, University of Michigan, 2013.
- American Heart Association Predoctoral Fellowship, American Heart Association, 2013
- Baxter Young Investigator Award for distinguished research for critical care therapies and medical products, Baxter Healthcare Corporation, 2013
- Best Annual Speaker Award, Microfluidics in Biomedical Sciences Seminar Series of 2012-2013, University of Michigan, 2013.
- Outstanding Paper Award, 2nd ASME Global Congress on NanoEngineering for Medicine and Biology, Boston, MA, 2013.
- Best Poster Award, 7th Annual UM Engineering Graduate Symposium, University of Michigan, 2012.
Publications
Textbook
- Raymond H.W. Lam and Weiqiang Chen, Biomedical Devices: Materials, Design, and Manufacturing, Springer Nature, 2019. ISBN 978-3-030-24236-7
Journal Articles
- Weiyi Qian, Tarik Hadi, Michele Silvestro, Xiao Ma, Cristobal F. Rivera, Apratim Bajpai, Rui Li, Zijing Zhang, Hengdong Qu, Rayan Sleiman Tellaoui, Annanina Corsica, Ariadne L. Zias, Karan Garg, Thomas Maldonado, Bhama Ramkhelawon, Weiqiang Chen, Microskeletal stiffness promotes aortic aneurysm by sustaining pathological vascular smooth muscle cell mechanosensation via Piezo1, Nature Communications, 2022, 13, 512.
- Rui Li, Walida Ali, Chao Ma, Apratim Bajpai, Ngoc Luu, Aarushi Varshney, Camden Riley Rowe, Weiqian Chen*, Surface presentation of the noncanonical Wnt5a motif to cytotoxic CD8+ T-cells promotes their mechanotransduction and activation, Chemical Communications, 2021, 57, 12667-12670.
- Chuanyu Wang, Chung-Hui Huang, Zhuangqiang Gao, Jialiang Shen, Jiacheng He, Alana MacLachlan, Chao Ma, Ya Chang, Wen Yang, Yuxin Cai, Yang Lou, Siyuan Dai, Weiqiang Chen, Feng Li, Pengyu Chen, Nanoplasmonic Sandwich Immunoassay for Tumor-Derived Exosome Detection and Exosomal PD-L1 Profiling, ACS Sensors, 2021, 6, 3308-3319.
- Mei ElGindi, Jiranuwat Sapudom, Ibrahim Hamed Ibrahim, Mohamed Al-Sayegh, Weiqiang Chen, Anna Garcia-Sabaté, Jeremy Teo, May the Force Be with You (Or Not): The Immune System under Microgravity, Cells, 2021, 10, 8, 1941.
- Zhuoyu Zhang, Lunan Liu, Chao Ma, Xin Cui, Raymond H. W. Lam, Weiqiang Chen, An in silico glioblastoma microenvironment model dissects the immunological mechanisms of resistance to PD-1 checkpoint blockade immunotherapy, Small Methods, 2021, 2100197.
- Apratim Bajpai, Rui Li, Weiqian Chen, The Cellular Mechanobiology of Aging: From Biology to Mechanics, Annals of the New York Academy of Sciences, 2020, 1491, 3-24.Chao Ma, Yansong Peng, Hongtong Li, Weiqiang Chen, Organ-on-a-Chip 2.0: Fitting into the drug development pipeline, Trends in Pharmacological Sciences, 2020, 42, 2, 119-133.
- Rui Li, Chao Ma, Haogang Cai, Weiqian Chen, The CAR T-cell Mechanoimmunology at a Glance, Advanced Science, 2020, 2002628.
- Xin Cui, Jie Tong, Jimmy Yau, Apratim Bajpai, Jing Yang, Yansong Peng, Mrinalini Singh, Weiyi Qian, Xiao Ma, Weiqian Chen, Mechanical forces regulate vascular cell asymmetric alignment, Biophysical Journal, 2020, 119, 1771-1780.
- Chao Ma, Matthew T. Witkowski, Jacob Harris, Igor Dolgalev, Sheetal Sreeram, Weiyi Qian, Jie Tong, Xin Chen, Iannis Aifantis, Weiqian Chen, Leukemia-on-a-Chip: Dissecting the chemo-resistance mechanisms in B-cell acute lymphoblastic leukemia bone marrow niche, Science Advances, 2020, 6, eaba5536.
- Xin Cui, Chao Ma, Varshini Vasudevaraja, Jonathan Serrano, Jie Tong, Yansong Peng, Michael Delorenzo, Guomiao Shen, Joshua Frenster, Renee-Tyler Tan Morales, Weiyi Qian, Aristotelis Tsirigos, Andrew S. Chi, Rajan Jain, Sylvia C. Kurz, Erik P. Sulman, Dimitris Placantonakis, Matija Snuderl, Weiqiang Chen, Dissecting the heterogeneity of immunosuppressive tumor microenvironments in a Glioblastoma-on-a-Chip for optimized anti-PD-1 immunotherapy, eLife, 2020, 9, e52253.
- Matthew T Witkowski, Igor Dolgalev, Nikki A Evensen, Chao Ma, Tiffany Chambers, Kathryn G Roberts, Sheetal Sreeram, Yuling Dai, Anastasia N Tikhonova, Audrey Lasry, Chunxu Qu, Deqing Pei, Cheng Cheng, Gabriel A Robbins, Joanna Pierro, Shanmugapriya Selvaraj, Valeria Mezzano, Marla Daves, Philip J Lupo, Michael E Scheurer, Cynthia A Loomis, Charles G Mullighan, Weiqiang Chen, Karen R Rabin, Aristotelis Tsirigos, William L Carroll, Iannis Aifantis, Extensive remodeling of the immune microenvironment in B cell acute lymphoblastic leukemia. Cancer Cell, 2020;37(6):867-882.e12.
- Muhammedin Deliorman, Farhad K. Janahi, Pavithra Sukumar, Roaa Alnemari, Samar Fadl, Ayoub Glia, Weiqiang Chen, Mohammad A. Qasaimeh*, A microfluidic-atomic force microscopy platform for the capture and mechanical characterization of circulating tumor cells in prostate cancer, Microsystems & Nanoengineering, 2020, 6, 20.
- Apratim Bajpai, Jie Tong, Weiyi Qian, Yansong Peng, Weiqiang Chen, The interplay between cell-cell and cell-matrix forces regulates cell migration dynamics, Biophysical Journal, 2019, 117, 1-10.
- Pavithra Sukumar, Muhammedin Deliorman, Ayoola T. Brimmo, Roaa Alnemari, Deena Elsori, Weiqiang Chen, Mohammad A. Qasaimeh, Airplug-mediated isolation and centralization of single T cells in rectangular microwells for biosensing, Advanced Therapeutics, 2019, 1900085.
- Qianbin Wang, Weiyi Qian, Xiaoyu Xu, Apratim Bajpai, Kevin Guan, Zijing Zhang, Roy Chen, Vittoria Flamini, and Weiqiang Chen, Energy-mediated machinery drives cellular mechanical allostasis, Advanced Materials, 2019, 1900453.
- Weiyi Qian and Weiqiang Chen, Probing single-cell mechanical allostasis using ultrasound tweezers, Cellular and Molecular Bioengineering, 2019, s12195-019-00578-z.
- Yuxin Cai, Jingyi Zhu, Jiacheng He, Yang Wen, Weiqiang Chen, and Pengyu Chen, Magnet Patterned Superparamagnetic Fe3O4/Au Core-Shell Nanoplasmonic Sensing Array for Label-Free High Throughput Cytokine Immunoassay, Advanced Healthcare Materials, 2019, 8, 1801478.
- Weiqiang Chen, Steven G. Allen, Weiyi Qian, Zifeng Peng, Shuo Han, Xiang Li, Yubing Sun, Chelsea Fournier, Liwei Bao, Raymond H.W. Lam, Sofia D. Merajver, and Jianping Fu, Biophysical Phenotyping and Modulation of ALDH+ Inflammatory Breast Cancer Stem-like Cells, Small, 2019, 1802891.
- Haoyu Wang, Renee-Tyler Tan Morales, Xin Cui, Jiongxian Huang, Weiyi Qian, Jie Tong, Weiqiang Chen, A Photoresponsive Hyaluronan Hydrogel Nanocomposite for Dynamic Immunomodulation, Advanced Healthcare Materials, 2018, 1801234.
- Ini-Isabée Witzel, Rasha Nasser, Anna Sabate, Jiranuwat Sapudom, Chao Ma, Weiqiang Chen, Jeremy CM Teo, Deconstructing immune microenvironments of lymphoid tissues for reverse engineering, Advanced Healthcare Materials, 2018, 1801126.
- Jingyi Zhu, Jiacheng He, Michael Verano, Ayoola T. Brimmo, Ayoub Glia, Mohammad A. Qasaimeh, Pengyu Chen, Jose O. Aleman, Weiqiang Chen, An Integrated Adipose-Tissue-On-Chip Nanoplasmonic Biosensing Platform for Investigating Obesity-associated Inflammation, Lab on a Chip, 2018.
- Xin Cui, Renee-Tyler Tan Morales, Weiyi Qian, Haoyu Wang, Jean-Pierre Gagner, Igor Dolgalev, Dimitris Placantonakis, David Zagzag, Luisa Cimmino, Matija Snuderl, Raymond H. W. Lam*, Weiqiang Chen, Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis, Biomaterials, 2018, 161, 164-178.
- Weiqiang Chen, Shuo Han, Weyi Qain, Shinuo Weng, Haiou Yang, Luis G. Villa-Diaz, Yubing Sun, Paul H. Krebsbach, Jianping Fu, Nanotopography regulates motor neuron differentiation of human pluripotent stem cells, Nanoscale, 2018, 10, 3556-3565.
- Xin Cui, Ya Liu, Dinglong Hu, Weiyi Qian, Dong Sun, Weiqiang Chen, Raymond H. W. Lam, A fluorescent microbead-based microfluidic immunoassay chip for immune cell cytokine secretion quantification, Lab on a Chip, 2018, 18, 522-531.
- W. Qian, L. Gong, X. Cui, Z. Zhang, A. Bajpai, C. Liu, A. B. Castillo, J. C. M. Teo, W. Chen, Nanotopographic Regulation of Human Mesenchymal Stem Cell Osteogenesis, ACS Applied Materials & Interfaces, 2017, 9, 41794-41806.
- S. Hill, W. Qian, W. Chen, J. Fu, Surface micromachining of polydimethylsiloxane for microfluidics applications, Biomicrofluidics, 2016, 10, 054114.
- W. Chen, S. G. Allen, A. K. Reka, W. Qian, S. Han, J. Zhao, L. Bao, V. Keshamouni, S. D. Merajver, J. Fu, Nanoroughened adhesion-based capture of circulating tumor cells with heterogeneous molecular expression and metastatic characteristics, BMC Cancer, 2016, 16, 614.
- C. Liu, X. Cui, T. M. Ackermann, V. Flamini, W. Chen, A. B Castillo, Osteoblast-Derived Paracrine Factors Regulate Angiogenesis in Response to Mechanical Stimulation, Integrative Biology, 2016, 8, 785-794.
- S. Weng, Y. Shao, W. Chen, J. Fu, Mechanosensitive subcellular rheostasis drives emergent single-cell mechanical homeostasis, Nature Materials, 2016, 15, 961-967.
- S. Hu, G. Liu, W. Chen, X. Li, W. Lu, R. H. W. Lam, J. Fu, Multiparametric biomechanical and biochemical phenotypic profiling of single cancer cells using elasticity microcytometer, Small, 2016, 12, 2300-2311.
- Y. Zhang, A. Gorden, W. Qian, W. Chen, Engineering Nanoscale Stem Cell Niche: Direct Stem Cell Behavior at Cell–Matrix Interface, Advanced Healthcare Materials, 2015, 4, 1900-1914.
- W. Qian, Y. Zhang, W. Chen, Capturing Cancer: Emerging Microfluidic Technologies for the Capture and Characterization of Circulating Tumor Cells, Small, 2015, 11, 3850-3872.
- W. Qian, Y. Zhang, W. Chen, Nanotopographic biomaterials for isolation of circulating tumor cells, Journal of Nanotechnology in Engineering and Medicine, 2014, 5, 040901.
- W. Chen, Y. Shao, X. Li, and J. Fu, Nanotopograghical surfaces for stem cell fate control: Engineering mechanobiology from the bottom, Nano Today, 2014, 9, 759-784.
- X. Li, W. Chen, G. Liu, W. Lu, and J. Fu, Continuous-flow microfluidic blood cell sorting for unprocessed whole blood using surface-micromachined microfiltration membranes, Lab on Chip, 2014, 14, 2565-2575.
- X. Li, W. Chen, Z. Li, L. Li, H. Gu, and J. Fu, Emerging microengineering tools for functional analysis and phenotyping of blood cells, Trends in Biotechnology, 2014, 32, 586-594.
- Y. Sun, K. M. Aw Yong, L. G. Villa-Diaz, X. Zhang, W. Chen, R. Philson, S. Weng, H. Xu, P. H. Krebsbach, and J. Fu, Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells, Nature Materials, 2014, 13, 599-604.
- B. Oh, N. Huang, W. Chen, J. Seo, P. Chen, T. T. Cornell, T. P. Shanley, J. Fu, and K. Kurabayashi, Integrated nanoplasmonic sensing in an optofluidic platform for human blood cellular functional immunoanalysis, ACS Nano, 2014, 8, 2667-2676.
- Y. Shao, J. M. Mann, W. Chen, and J. Fu, Global Architecture of F-actin cytoskeleton regulates cell shape-dependent endothelial mechanotransduction, Integrative Biology, 2014, 6, 300-311.
- W. Chen, N. Huang, B. Oh, R. H. W. Lam, R. Fan, T. T. Cornell, T. P. Shanley, K. Kurabayashi, and J. Fu, Surface-micromachined microfiltration membranes for efficient isolation and functional immunophenotyping of subpopulations of immune cells, Advanced Healthcare Materials, 2013, 2, 965-975.
- Z. Fan, Y. Sun, D. Chen, D. Tay, W. Chen, C. X. Deng, and J. Fu, Acoustic tweezing cytometry for live-cell subcellular control of intracellular cytoskeleton contractility, Scientific Reports, 3, 2176.
- A. Singh, S. Suri, T. Lee, J. M.Chilton, M. T. Cooke, W. Chen, J. Fu, S. L. Stice, H. Lu, T. C. McDevitt, and A. J. García. Adhesive signature-based, label-free isolation of reprogrammed human induced pluripotent stem cells. Nature Methods, 2013, 10, 438-444.
- W. Chen, N. Huang, X. Li, Z. T. Yu, K. Kurabayashi and J. Fu. Emerging microfluidic tools for functional immunophenotyping: A new potential paradigm for immune status characterization. Frontiers in Oncology, 2013, 3, 98.
- W. Chen, S. Weng, F. Zhang, S. Allen, X. Li, L. Bao, R. H. W. Lam, J. A. Macoska, S. D. Merajver, and J. Fu, Nanoroughened surfaces for efficient capture of circulating tumor cells without using capture antibodies, ACS Nano, 2013, 7, 566-575.
- W. Chen, Y. Sun and J. Fu, Microfabricated nanotopological surfaces for study of adhesion-dependent cell mechanosensitivity, Small, 2013, 9, 81-89.
- W. Chen, R. H. W. Lam and J. Fu, Photolithographic surface micromachining of polydimethylsiloxane (PDMS), Lab on a Chip, 2012, 12, 391-395.
- N. Huang, W. Chen, B. Oh, T. Cornell, T. Shanley, J. Fu and K. Kurabayashi, An integrated microfluidic platform for in-situ cellular cytokine secretion immunophenotyping, Lab on a Chip, 2012, 12, 4093-4101.
- W. Chen, L. G. Villa-Diaz, Y. Sun, S. Weng, R. H. W. Lam, L. Han, R. Fan, P. H. Krebsbach, and J. Fu, Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells, ACS Nano, 2012, 6, 4094-4103.
- Y. Sun, L. G. Villa-Diaz, R. H. W. Lam, W. Chen, P. H. Krebsbach and J. Fu, Matrix mechanics regulates fate decisions of human embryonic stem cells, PLoS ONE, 2012, 7, e37178.
- R. H. W. Lam, Y. Sun, W. Chen and J. Fu, Elastomeric microposts integrated into microfluidics for flow-mediated endothelial mechanotransduction analysis, Lab on a Chip, 2012, 12, 1865-1873.
- Y. Wu, Y. Lu, W. Chen, J. Fu and R. Fan, In silico experimentation of glioma microenvironment development and anti-tumor therapy, PLoS Computational Biology, 2012, 8, e1002355.
- W. Chen, K. P. Chen, M. D. Thoreson, A. V. Kildishev and V. M. Shalaev, Ultrathin, ultrasmooth and low-loss silver films via wetting and annealing, Applied Physics Letter, 2010, 97, 21107.
- W. Chen, M. D. Thoreson, A. V. Kildishev and V. M. Shalaev, Fabrication and Optical Characterizations of Smooth Silver-Silica Nanocomposite Films, Laser Physics Letters, 2010, 7, 677-684.
- W. Chen, M. D. Thoreson, S. Ishii, A. V. Kildishev and V. M. Shalaev, Ultra-thin ultra-smooth and low-loss silver films on a germanium wetting layer, Optics Express, 2010, 18, 5124-5134.
- Z. Su, G. Ding, W. Lu, W. Chen, Broadband circularly polarized CPW-fed slot antenna array for millimeter wave application, Microelectronics Journal, 2009, 40, 1192-1195.
- R. B. Nielsen, M. D. Thoreson, W. Chen, A. Kristensen, J. M. Hvam, V. M. Shalaev and A. Boltasseva, Toward superlensing with metal–dielectric composites and multilayers, Applied Physics B, 2010, 100, 93-100.
- W. Chen, G. Ding, B. Li, X. Sun, Design and simulation of MEMS-based broadband unidirectional CPW-fed rectangular slot antenna, Microsystem Technologies, 2008, 14, 343-347.
- W. Chen, G. Ding, Z. Su and B. Li, A novel MEMS-based CPW-fed slot antenna for broadband circular polarization and unidirectional radiation, Journal of Micromechanics and Microengineering, 2007, 17, 2352–2359.
- Z. Yang, G. Ding, W. Chen, S. Fu, X. Sun and X. Zhao, Design, simulation and characterization of inertia micro-switch fabricated by non-silicon surface micromachining, Journal of Micromechanics and Microengineering, 2007, 17, 1598–1604.
Research Grants
NSF Engineering of Biomedical Systems Grant (CBET-2103219)
Project Title: “Bioengineering Leukemic Bone Marrow Niche for Dissecting the Heterogeneous Leukemia Chemo-resistance Mechanisms”
Granting Agency: National Science Foundation
Principal Investigator: Weiqiang Chen
Award Period: 07/01/2021–08/30/2024
The Colton Center for Autoimmunity Innovative Grant
Project Title: “Impact of type I interferons on Rheumatoid arthritis patient monocytes, monocyte-derived macrophages and fibroblast-like synoviocytes using a novel vascularized pannus-on-a-chip model”
Granting Agency: The Colton Center for Autoimmunity
Principal Investigator: Weiqiang Chen & Theresa Wampler Muskardin
Award Period: 07/01/2021–06/30/2022
NIGMS Administrative Supplements for Equipment (3R35GM133646-02S1)
Project Title: “Purchase of Spinning Disk Confocal Microscope”
Granting Agency: National Institute of General Medical Sciences
Principal Investigator: Weiqiang Chen
Award Period: 07/01/2020-06/30/2021
Innovative Research in Cancer Nanotechnology (1R01CA243001-01)
Project Title: “Map Leukemia-immune Cell Communication with Nanoplasmon Ruler in CAR T-Cell Immunotherapy”
Granting Agency: National Cancer Institute
Principal Investigator: Weiqiang Chen & Pengyu Chen (Auburn University)
Award Period: 07/01/2020-06/30/2025
NIGMS Maximizing Investigators' Research Award for Early Stage Investigators (1R35GM133646-01) Project Title: “Dissecting and Engineering CAR T-cell Function for Optimized Immunotherapy”
Granting Agency: National Institute of General Medical Sciences
Principal Investigator: Weiqiang Chen
Award Period: 09/01/2019-06/30/2024
NIBIB Trailblazer Award (1R21EB025406)
Project Title: “Engineered Glioblastoma Tumor Immunity for Personalized Immunotherapy”
Granting Agency: National Institute of Biomedical Imaging and Bioengineering
Principal Investigator: Weiqiang Chen & Matija Snuderl (NYU Department of Pathology)
Award Period: 09/01/2018–05/31/2022
NSF Nano-biosensing Grant (CBET-1701322 & CBET-1701363)
Project Title: “Collaborative Research: Plasmofluidic Nanoantenna-Superlens Biosensor for Single-Cell Functional Immunophenotyping”
Granting Agency: National Science Foundation
Principal Investigator: Weiqiang Chen & Pengyu Chen (Auburn University)
Award Period: 09/01/2017–08/31/2020
AHA Scientist Development Grant (16SDG31020038)
Project Title: “Nanotopography-Dependent Neural Stem Cell Induction of Human Induced Pluripotent Stem Cells for Post Stroke Neural Rehabilitation”
Granting Agency: American Heart Association
Principal Investigator: Weiqiang Chen
Award Period: 07/01/2016–06/30/2019
NYUAD Research Enhancement Fund
Project Title: “Single Cell Capture and Nanomechanical Phenotyping of Prostate Circulating Tumor Cells using a Novel Microfluidic Platform”
Granting Agency: New York University Abu Dhabi
Principal Investigator: Mohammad Qasaimeh (NYU Abu Dhabi) & Weiqiang Chen
Award Period: 09/01/2017–08/31/2019
New York University Mega-grant Initiative Grant
Project Title: “Bioengineer in vitro Glioma Vascular Microenvironment Model”
Granting Agency: New York University Provost Office
Principal Investigator: Weiqiang Chen
Award Period: 04/01/2017–03/31/2018
New York University Whitehead Fellowship for Junior Faculty in Biomedical and Biological Sciences
Project Title: “Glioma-on-a-Chip for in vitro Validation of Personalized PD-1/PD-L1 Immunotherapy”
Granting Agency: New York University
Principal Investigator: Weiqiang Chen
Award Period: 09/01/2017–08/31/2018
New York University CTSI Pilot Project
Project Title: “Microfluidic Functional Immunophenotyping of Human Adipose Tissue Immune Cells”
Granting Agency: New York University
Principal Investigator: José O. Alemán (NYU Department of Medicine) & Weiqiang Chen
Award Period: 04/01/2017–03/31/2018
New York University Global Seed Grants for Collaborative Research
Project Title: “Plasmonic-based Microfluidic Biosensor for Immune Cell Phenotyping”
Granting Agency: New York University
Principal Investigator: Weiqiang Chen & Mohammad Qasaimeh (NYU Abu Dhabi)
Award Period: 04/01/2016–03/31/2018
New York University Research Challenge Fund
Project Title: “Nanotopography Mediated Neural Differentiation of Human Induced Pluripotent Stem Cells for the Post Stroke Rehabilitation of Neurological Impairments”
Granting Agency: New York University
Principal Investigator: Weiqiang Chen
Award Period: 06/01/2015–05/31/2016
Research News
Leukemia-on-a-chip: Dissecting the chemoresistance mechanisms in B cell acute lymphoblastic leukemia bone marrow niche
Weiqiang Chen, associate professor of biomedical and mechanical and aerospace engineering led this research.
B cell acute lymphoblastic leukemia (B-ALL) hijacks the bone marrow (BM) microenvironment to form leukemic BM “niches,” facilitating chemoresistance and, ultimately, disease relapse. The key to developing more effective, targeted therapies depends on researchers' ability to isolate and examine with these evolving, heterogeneous interactions among distinct B-ALL subtypes and their varying BM niches. Current in vivo methods limit that ability.
A team including researchers from the NYU Tandon School of Engineering's departments of Mechanical and Aerospace, and Biomedical Engineering, and from NYU Langone's Perlmutter Cancer Center and Department of Pathology demonstrated an in vitro organotypic “leukemia-on-a-chip” model to emulate the in vivo B-ALL BM pathology and comparatively studied the spatial and genetic heterogeneity of the BM niche in regulating B-ALL chemotherapy resistance.
In the study "Leukemia-on-a-chip: Dissecting the chemoresistance mechanisms in B cell acute lymphoblastic leukemia bone marrow niche," published in ScienceAdvances, the team used their system to describe the heterogeneous chemoresistance mechanisms across various B-ALL cell lines and patient-derived samples, showing that the leukemic perivascular, endosteal, and hematopoietic niche-derived factors maintain B-ALL survival and quiescence. Furthermore, they demonstrated the preclinical use of their lab-on-a-chip model to test niche-cotargeting regimens, which may translate to patient-specific therapy screening and response prediction.
This work was supported by the National Science Foundation, the U.S. National Institutes of Health, the Leukemia & Lymphoma Society, the New York State Department of Health (NYSTEM Program), and the St. Baldrick’s Cancer Research Foundation (I.A).
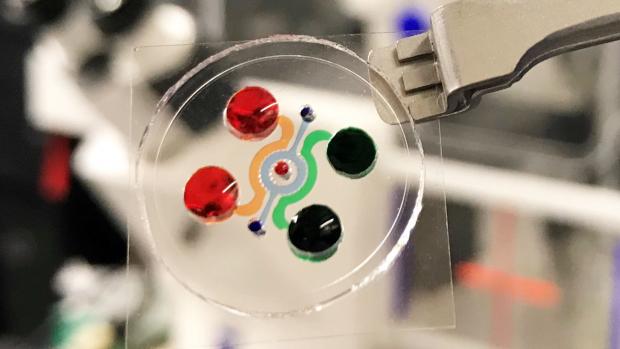
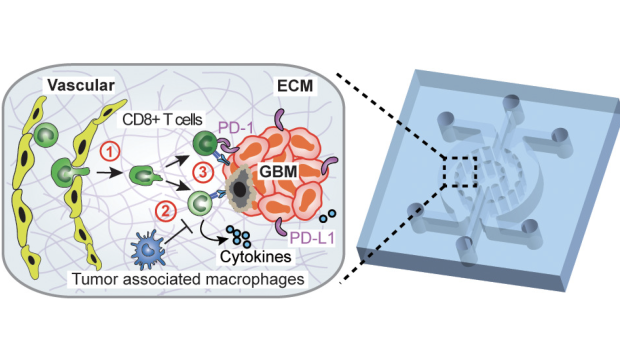
Dissecting the immunosuppressive tumor microenvironments in Glioblastoma-on-a-Chip for optimized PD-1 immunotherapy
Weiqiang Chen, associate professor of biomedical and mechanical and aerospace engineering led this research.
Glioblastoma (GBM) is the most common and aggressive primary brain tumor among adults, with an average survival of less than 14 months despite aggressive surgery, chemotherapy, and radiotherapy. One of the most promising therapies has been the inhibition of programmed cell death protein-1 (PD-1), which can turn the immune system against the tumors in order to destroy it. However, that approach has been difficult due to the genetic difference between blastomas and the environments they form in.
Improving the clinical use of anti-PD-1 immunotherapy in GBM patients requires a comprehensive understanding of tumor genetics and microenvironment as well as the ability to dissect the dynamic interactions among GBM and immune suppressor cells. Now, a team of researchers led by NTU Tandon’s Weiqiang Chen have integrated critical markers of these microenvironments in a microfluidics-based ex vivo microphysiological system termed ‘GBM-on-a-Chip.’
Modeling the human immune environment in the current animal-based cancer models is challenging. Compared to chemotherapy, it is difficult to preclinically validate and study immunotherapy. Discrepancies between preclinical and clinical results have raised concerns about how the findings from the current models can be translated to patients. The engineered tumor model on chips can be an alternative to the current animal models and patient studies, and even achieve a so-called "clinical trial on chips" for a pre-screening of patients suitable for immunotherapy, and screening personalized therapy for each patient. These chips are patient-specific, allowing longitudinal analysis of cells to understand how the environment around a tumor changes the way that PD-1 cells act.
The team – including researchers from NYU Tandon, NYU Langone Health and NYU School of Medicine, with funding from the National Science Foundation and National Institutes of Health – were able to use the results of this longitudinal study to show that molecularly distinct GBM subtypes have distinct epigenetic and immune signatures that may lead to different immunosuppressive mechanisms. They could see which cells were elevated past their healthy amounts, and which were not responding as they should. This allowed them to administer a specialized treatment for the specific environment, supressing some cells like tumor-associated macrophages while boosting the effectiveness of specific T-cells. Thus, they showed that these patient-specific chips could lead to personalized immunotherapy screening, potentially improving therapeutic outcomes in GBM patients.
The research was conducted with investigators from NYU Tandon, NYU Langone Health, and the NYU School of Medicine.