National Institutes of Health supports new research that could lead to personalized cancer treatments
Weiqiang Chen, assistant professor of biomedical engineering and mechanical and aerospace engineering at the NYU Tandon School of Engineering, and a member of the NYU Langone Laura and Isaac Perlmutter Cancer Center, wants to help harness the power of cytokines to treat cancer. He has received his second major grant from the National Institutes of Health (NIH) in under a year, both involving research focused on insights that could optimize modified T-Cell lymphocytes for the immunotherapeutic treatment of cancer.
The new research, “Map Leukemia-immune Cell Talks with Nanoplasmon Ruler in CAR T-Cell Immunotherapy,” is supported by a $2.6 million grant from the NIH’s National Cancer Institute. The work will expand upon Chen’s five-year, $1.7 million NIH Outstanding Investigator Award of last fall.
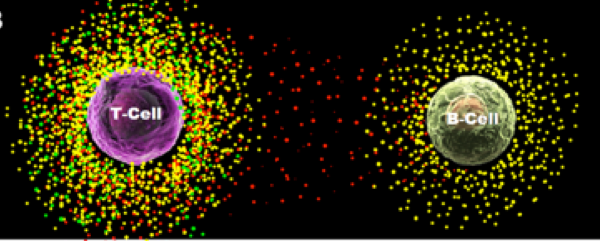
Visualizing a single cell cytokine communication using nanoplasmon ruler technique
The team, including Iannis Aifantis at the NYU Grossman School of Medicine, Pengyu Chen of Auburn University, and Feng Ding from Clemson University, will develop a new biosensor to examine T-cells engineered with special proteins called chimeric antigen receptors (CAR). These lymphocytes are able to target biomarkers for B cells, including neoplastic mutations typical of acute lymphoblastic leukemia. The goal is to understand factors that hinder cytokine signaling, or “crosstalk” between these CAR-T cells during this process. The results could make it possible for clinicians to design personalized treatments for individual patients.
Chen, who heads NYU Tandon’s Applied Micro-Bioengineering Laboratory, said his research aims to provide insights that can help pharmacologists and physicians “tune” this powerful autoimmune response to attack malignant cells.
“Immune cells are highly diverse and each subtype has a different role in immune response,” said Chen, pointing out that even individual cells may behave differently, making it challenging for clinicians to tease out the most effective use of immune cells in immunotherapy for a given cancer, patient, target cell, or tumor location, among many other variables.
“In cancer immunotherapy, immune cells experience rapid dynamic alterations and show cell-to-cell variability across the same type or different types of cell groups in their functions,” he explained. “The numbers, proportions, and functional responses of each subset changes drastically in immunotherapy. This complex heterogeneity of immune cells, and their highly dynamic and interdependent nature are major hurdles to identify key parameters for effective therapeutic intervention.”
The team will perform real-time and traceable monitoring of the location and timing of cytokine secretion to develop a mechanistic understanding of how CAR T-cells initiate, activate, communicate and respond, an experimental capability lacking within existing clinical practices, which use “bulk assay” methods primarily based on measurements that are static because they don’t shed light on the intimate physical interactions between specific cells over time.
“There is an emerging need for platforms that allow direct visualization and mapping of cytokine production, diffusion, transportation for better understanding CAR T-cells and immune cell communications,” he said. “That is what we are designing.”
To study these processes, the team will develop a system comprising a sensor called a nanoplasmon ruler-microwell array platform, which uses plasmonic gold nanoparticles conjugated by DNA in a setup that shows great potential in detecting cytokines secreted by single cells in real-time. In order to study how two single immune cells communicate to each other through cytokine signals, the team will integrate this platform on a microfluidic microwell array chip that can isolate tens of thousands of single-cell pairs and integrate them with the nanoplasmon ruler sensor. By doing so the researchers will be able to map single-cell cytokine crosstalk in a highly efficient, high-throughput manner. This single-cell sensing technique for monitoring immunotherapeutic processes could allow screening of optimal, effective and safe therapy.
In 2018 Chen received the National Institute of Biomedical Imaging and Bioengineering Trailblazer Award for a lab-on-a-chip system for testing immunotherapy for glioblastoma, and last year he was one of just 12 researchers to be named a 2019 Young Innovator by the Biomedical Engineering Society.


